The dissolution and precipitation of carbonates are critically important processes in a wide array of natural and engineering processes such as the global carbon cycle, Karst topography, biomineralization, rock weathering, geological carbon sequestration, petroleum reservoir acidizing. A better understanding of how carbon is mineralized into and released from solid mineral phases would contribute to the decarbonization of our economy and society.
Importantly, most often these dissolution and precipitation processes occur not in open waters, but in microscale confinements, such as the pore spaces of rocks and soils and the intra- and extra-cellular spaces inside marine organisms. We still lack a comprehensive understanding of how the dissolution and precipitation dynamics of carbonates interact with small spatial confinement. There are many details remaining unexplored.
Microfluidics offers an ideal tool for addressing this challenge. Microfluidics is the lab-on-a-chip technology of controlling/manipulating small amounts of fluids, typically from micro liter to nano liter, for the investigations of small-scale phenomena related to flow, reaction, phase change, material synthesis, cellular interactions etc. It has the capability of direct optical visualization such that complex physicochemical processes can be studied in situ and dynamically. Glass is the widely used material for fabricating microfluidic chips, partly because of its chemical resistance to most chemicals and low cost, which unfortunately, limits it applicability to reactive problems such as rock dissolution.
We developed novel fluid mixing techniques to precipitate calcium carbonate minerals in glass microfluidic chips. These precipitated minerals can either be sporadic particles confinement in a microfluidic chamber or form an entire carbonate porous medium. Afterwards, acid can be injected into the chip to dissolve the minerals or porous media.
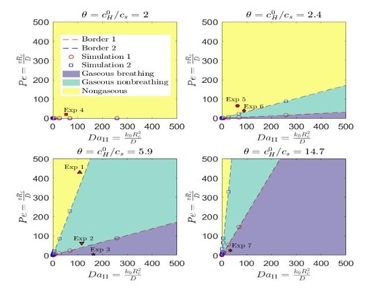
Utilizing our microfluidic platforms, we discovered novel regimes of carbonate dissolution confined in a microchamber, characterized by fluctuating and non-fluctuating patterns of the produced CO2 phase and if there is CO2 produced. We generated phase diagrams that describe the distribution of these regimes through numerical simulations. The following figures show experimental images of the three regimes and the phase diagrams of them based on Peclet number Pe, Damkohler number Da, and dimensionless acid concentration θ.
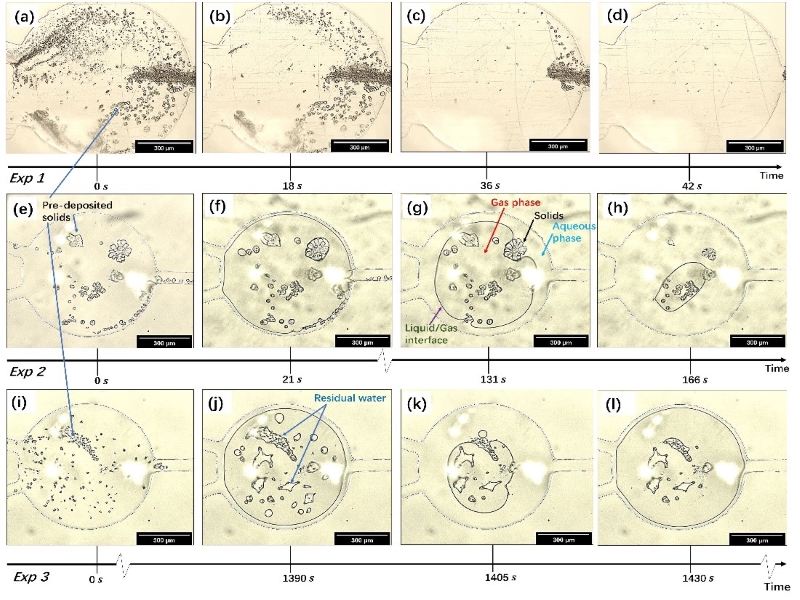
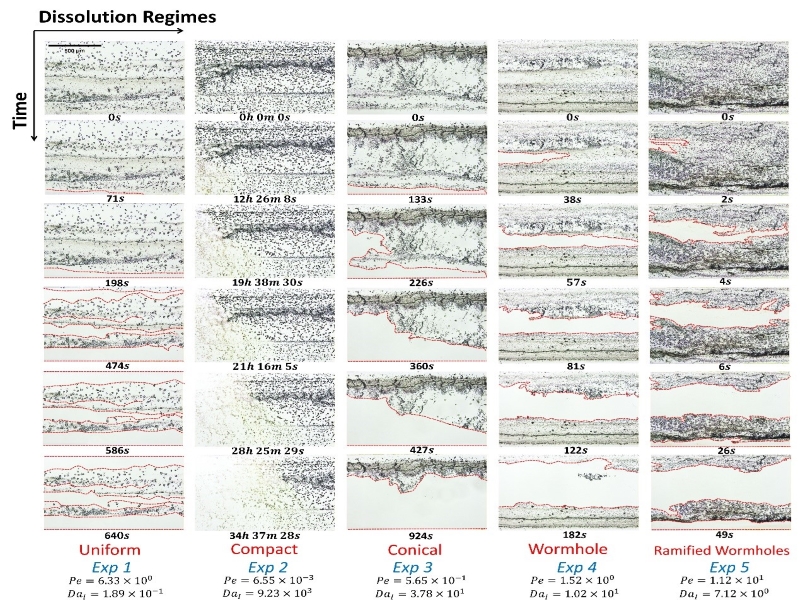
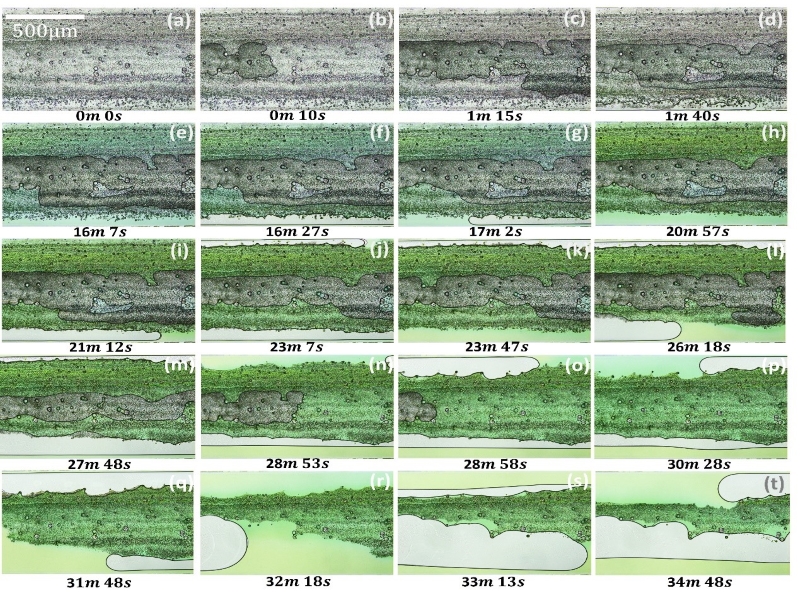
We also created carbonate porous media in a microfluidic channel and use it to study single- and multi-phase dissolution of carbonate rocks. In single-phase dissolution only aqueous phase are in the pore spaces while in the multiphase dissolution there are also nonaqueous phases such as CO2. The following figures show experimental images of single-phase dissolution and multi-phase dissolution where CO2 is present.
Utilizing the microfluidic platforms, we also discovered that the particle size statistics of carbonates during its precipitation in confinement is a power-law distribution, rather than the bimodal distribution formed in open waters. We proposed a general model, named the extended Yule process to describe the power-law pattern and achieved excellent agreement with experimental data. The following figure shows that the statistics transits from power-law to bimodal when confinement is relaxed.
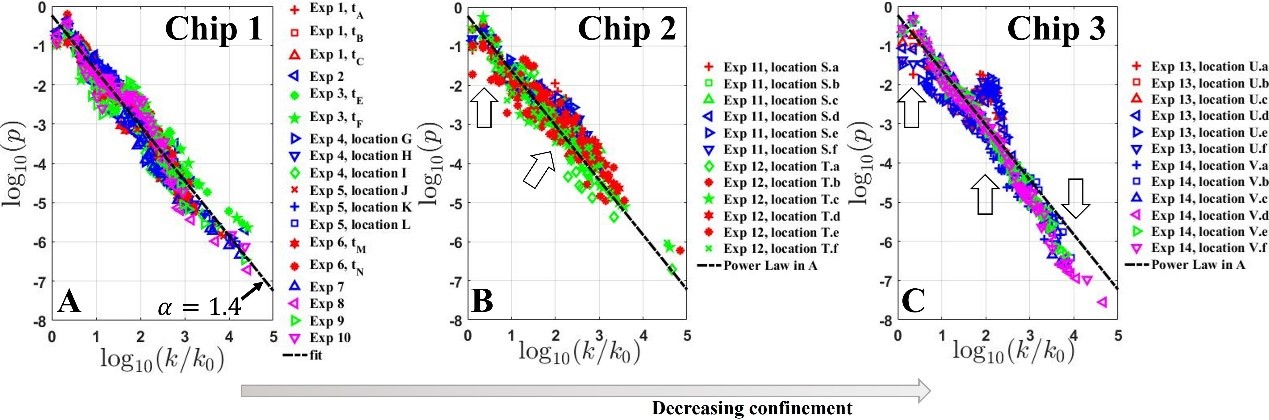
Our works show that small confinement generate many interesting patterns of carbonates dissolution and precipitation that is not possible in bulk solutions. The microfluidic methodology of generating carbonate reactive system in glass microfluidic chips provides an inexpensive and efficient solution to reactive transport problems.
References
1. Jianping Xu and Matthew T Balhoff. Emergence of Power Law Particle Size Distribution in Microfluidic Calcium Carbonate Precipitation: An Extended Yule Process with a Ripening Effect, Physical Review Letters, (2023), in review.
2. Jianping Xu and Matthew T Balhoff. Dissolution-After-Precipitation (DAP): A Simple Microfluidic Approach for Studying Carbonate Rock Dissolution and Multiphase Reactive Transport Mechanisms, Lab on a Chip 22, 4205-4223 (2022).
3. Jianping Xu and Matthew T Balhoff. Novel regimes of calcium carbonate dissolution in micron-scale confined spaces, Advances in Water Resources 164, 104200 (2022).


