Energi Simulation IAP on Carbon Utilization and Storage
PI: Ryosuke Okuno (Email:
Overview
This IAP is an interdisciplinary platform for fundamental and applied research on sustainable energy ecosystems, including subsurface energy resources and carbon capture, utilization, and storage (CCUS). In this IAP spotlight page, three projects are highlighted among other projects within the IAP, aiming to address techno-economic-environmental challenges in carbon management.

- CO2 Capture as Bicarbonate
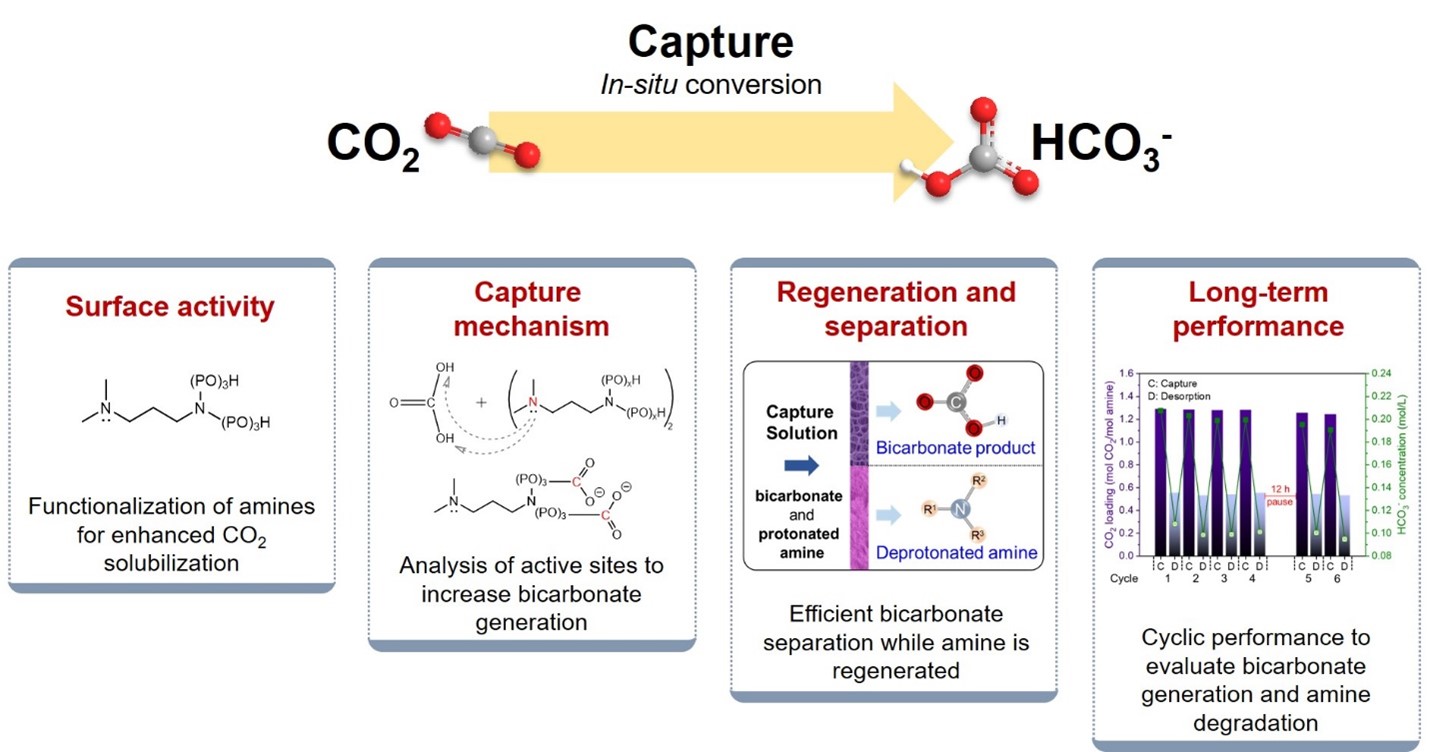
As part of CCUS, amine-based post-combustion capture has been commercially used, but it is energy-intensive to strip the CO2 captured by conventional amine solutions as carbamates while regenerating the amine solvent by heating it to a high temperature (e.g., 120 – 180°C). The regeneration step accounts for a large fraction of the total operating cost. Also, the traditional method of CO2 “catch-and-release” is unnecessary when the downstream processes in carbon management do not require CO2 as a carbon carrier. Many green chemicals can be generated from bicarbonate in aqueous media at lower costs and carbon intensity than from CO2.
This project is to evaluate CO2 capture as bicarbonate, aiming to improve the overall efficiency of carbon management by not using CO2 as a carbon carrier. Two main components of the research are i) CO2 capture as bicarbonate using a novel class of surface-active tertiary amines and ii) the bicarbonate separation and amine regeneration in a single step. The overall objective of this research is to examine the energy efficiency of the bicarbonate pathway in comparison to the conventional CO2 stripping process that is enabled and thus burdened by the need for temperature swings.
- Formate Species as Carbon/Hydrogen Carriers
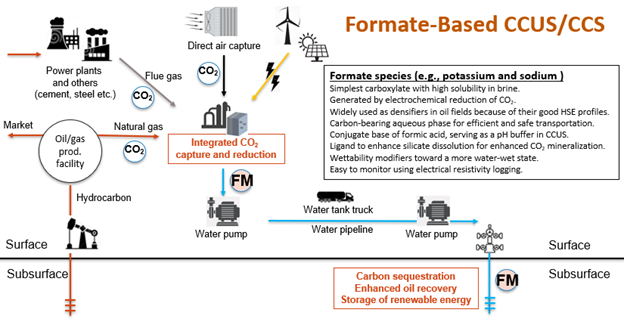
Conventional CCUS uses CO2 as a carbon carrier, which possesses various drawbacks related to its physical properties, such as low carbon density at low to moderate pressure, low mass density, low viscosity, immiscibility with water, and corrosivity. The potential risk of CO2 leakage from subsurface storage sites by the buoyancy-driven upward flux must be carefully monitored and controlled for decades. Also, the significant costs associated with CO2 capture, compression, transportation, and recycling after CO2 breakthrough at production wells are practically important factors for subsurface CO2 storage at scale.
Alkali formates can be used as an alternative to CO2 as a carbon carrier for geological storage that addresses the techno-environmental concerns mentioned above. Contrary to CO2, formate species (e.g., sodium and potassium) are highly soluble in brine with a carbon density of up to 18 mol/L (comparable to >12 MPa compressed CO2) even at standard conditions. In addition, they have been used as densifiers for drilling fluids in oil fields because of their health-safety-environmental profiles. Since formate species are stable under typical conditions of subsurface carbon storage, the formate-based carbon storage is analogous to, but more reliable than the solubility trapping mechanism of CO2 without involving less secure mechanisms, such as structural and capillary trapping. Moreover, aqueous formate solutions can be used as pH buffers in combination with or after CO2 injection, enabling geochemical control over the geological sealing integrity of CO2 storage sites. Also, formate species have been studied as wettability modifiers on carbonate rocks leading to potential applications for enhanced oil recovery (EOR).
This research project is to investigate the potential of an efficient and reliable geological carbon sequestration strategy using aqueous formate solution as a carbon carrier for CCUS. Although carbon storage in aquifers is the ultimate goal, injection of aqueous formate solution into an oil reservoir is also studied as an economically viable option for carbon storage without solely relying on the carbon tax credit. Such revenue sources from enhanced oil production can provide the time and resources to further optimize this technology which will facilitate the transition from increased oil production to low-cost, secure carbon sequestration. An aqueous formate solution with a moderate carbon density of 10 mol/L is equivalent to dissolving 70 kg of CO2 per barrel of brine. This is approximately 250 times the CO2 solubility in brine at standard conditions. If formate-based carbon storage was deployed by utilizing the produced water in oil fields in the lower 48 states, which amounts to at least 40 billion barrels per year, 2.8 G tonnes of CO2 equivalent carbon per year can be stored with the existing water-disposal sites and facilities.
- Aqueous Nanobubble Dispersion of CO2
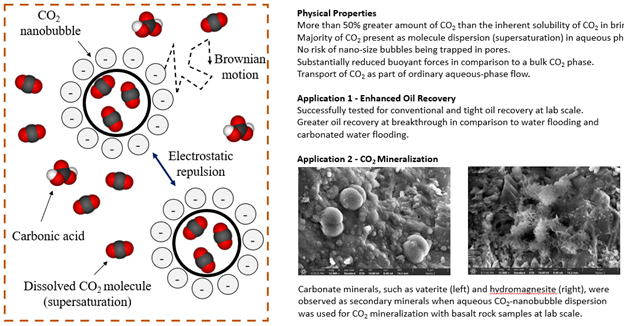
Carbonated water has been used as a CO2 injection method for EOR and geological carbon sequestration. It recently attracted attention particularly because carbonated water may accelerate the eventual mineralization of CO2 without needing the CO2 dissolution step, for example, in (ultra)mafic formations (e.g., basalt). An obvious shortcoming of using carbonated water is that the carbon density in carbonated water (i.e., CO2 solubility) is much smaller than pure CO2 at injection conditions (i.e., supercritical CO2), and the CO2 solubility decreases with salinity and temperature. An estimation based on CarbFix’s CO2 mineralization project is that 22 tonnes of water was required to capture 1 tonne of pure CO2 for storage; that is, the carbon density was approximately 1 mol/L, a common level of CO2 solubility in water.
It is possible to increase the CO2 content in water beyond carbonated water by generating nanobubbles of CO2 dispersed in water. Aqueous nanobubble dispersion refers to a state in which nano-scale bubbles of water-immiscible gaseous species are dispersed in the external water phase that is supersaturated by the gaseous species. Nanobubble dispersions have been applied in various industries, such as agriculture and wastewater treatment, with the ability to contain a large amount of gas. Kinetically stable nanobubbles at atmospheric pressure in an open system have been reported because of extremely small buoyant forces, repulsive forces among negatively charged surfaces of nanobubbles, and the supersaturated water suppressing Ostwald ripening. However, the previous studies on nanobubble dispersions are focused on an open system near atmospheric pressure. We have uniquely studied the fundamentals of nanobubble dispersions for subsurface applications including geological CO2 sequestration and CO2 EOR.


